BESREMi PEN


BESREMi is a prescription medicine that is used to treat adults with polycythemia vera
Get notified

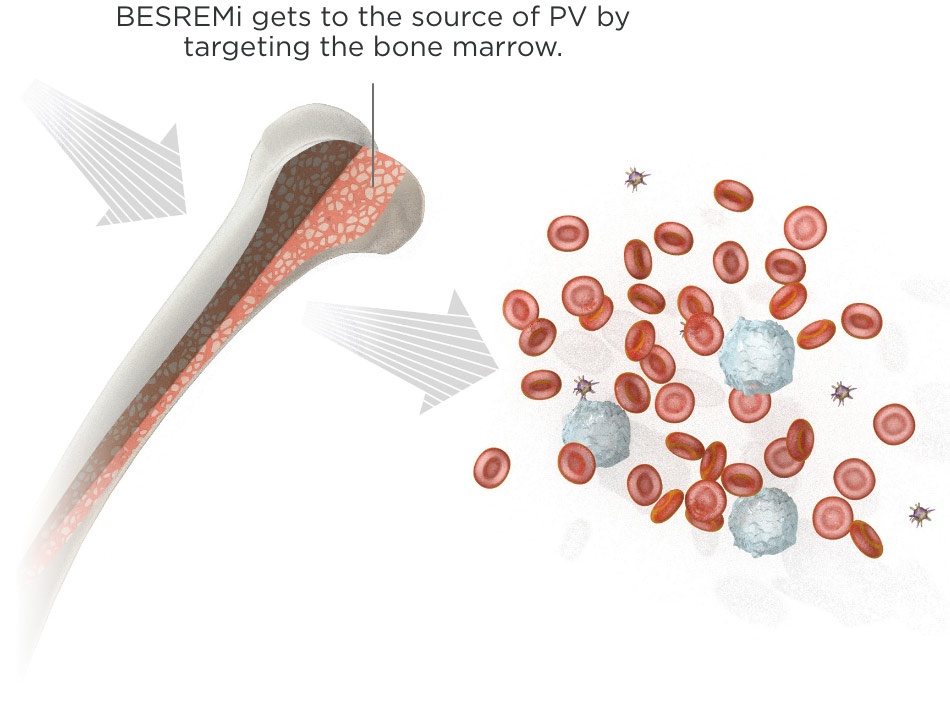
BESREMi targets PV at the source of the disease
Now is the time: wherever you are in your journey with polycythemia vera (PV), it's important that you understand your treatment options. Consider talking with your doctor about a treatment that targets PV at its source.
BESREMi is the only FDA-approved treatment indicated for adults with PV that targets the bone marrow, helping to control blood cell counts. That's what makes BESREMi different—it addresses the cause of PV.
 Red Blood Cell
Red Blood Cell
 White Blood Cell
White Blood Cell
 Platelet
Platelet
BESREMi is not chemotherapy
Ropeginterferon alfa-2b-njft (BESREMi) is a recommended treatment for certain patients with PV1
The National Comprehensive Cancer Network® (NCCN®) is a not-for-profit alliance of 33 leading cancer centers devoted to patient care, research, and education. The alliance creates national guidelines for recommended cancer treatment, called the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®).
The NCCN Guidelines® are:
- Designed to support decision-making
- A well-respected resource for doctors around the world
- Based on the latest data and clinical evidence
- Continuously updated and revised to reflect new information
These guidelines recommend ropeginterferon alfa-2b-njft (BESREMi) as a preferred first-line cytoreductive treatment option for certain patients with PV.1
BESREMi was proven to help a broad range of people living with PV
One study looked at the efficacy
People were included regardless of:
- History of cardiovascular eventsIncidents that can damage the heart and blood vessels, such as a heart attack or stroke.
- Prior treatment with hydroxyureaA type of chemotherapy that reduces the production of abnormal blood cells and lowers the risk of blood clots.(HU), a type of chemotherapy
Of the people who used BESREMi in the clinical study:
- All were 35 to 82 years of age
- 33% had been treated with HU
- 22% had a previous thrombotic eventSituations where blood clots form in a blood vessel or the heart, which can block blood flow.
BESREMi showed meaningful outcomes
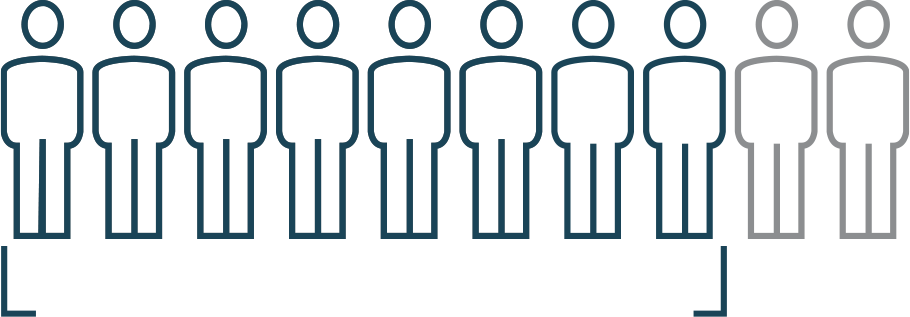

8 out of 10
achieved complete
hematologic response
- Blood cell counts (red blood cellsA common type of blood cell that delivers oxygen to the rest of the body., white blood cellsA type of blood cell that helps the body fight infections and disease., and plateletsSmall cell fragments that help form clots and stop bleeding.) returned to a normal level
- No phlebotomyA procedure of drawing blood to reduce excess blood cells and decrease blood volume.in the past 2 months
BESREMi displayed PV control over the long term
Complete hematologic response (CHR) was observed over a 7.5-year clinical trial.
Another study looked at the efficacy and safety of BESREMi in adults with PV over a period of 6 years.
People were included if they:
- Completed an initial study and benefited from BESREMi
- Had no history or less than 3 years of treatment with HU
Of the people who used BESREMi in the study:
- All were between 49 and 66 years old
- 32% had been treated with HU
- 22% had experienced a previous thrombotic event
BESREMi contributed to years without any phlebotomies
Phlebotomy-free rates
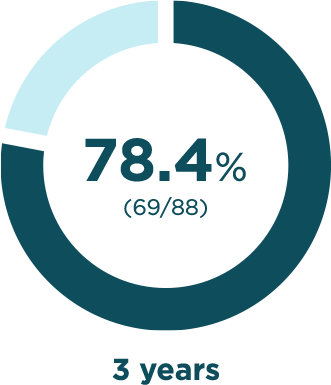

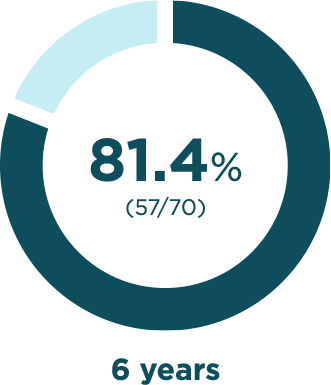
Percentage of participants in which no phlebotomies
were required to maintain normal hematocritThe percentage of red blood cells in your blood. levels*
levels*
*Among those with available data for each treatment year.
Taking charge of my treatment: Susan's story
Research and education may help you feel informed and empowered when exploring PV treatments. Watch Susan share how shaping her treatment plan with her doctor helped her discover BESREMi, which she chose as she continued her PV journey.

How to get started with BESREMi
BESREMi Pen™ is a single-dose prefilled pen that you administer once every 2 weeks. Find out how the ready-to-use pen delivers simplified dosing.